When Do Electrons Emit Energy
The movement of electrons around the nucleus and the energy levels Physics notes for high school: how energy of electrons is converted in Atomic spectra and models of the atom
Video: Electron Energy Level Transitions | Nagwa
Electron energy and light spectra Electron energy levels of atoms Electron transitions nagwa
Hydrogen spectra atom spectrum line bohr model chemistry emission absorption atomic atoms structure light electron energy photon when which electronic
Energy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustrationElectron spectroscopy absorption libretexts absorbs emission photon spectrum absorb simplified vibrational orbit chemical absorbance emits relaxation Science of fluorescence and fluorescent light photographyPhoton atom absorbs photons transitions hydrogen electron socratic wavelengths jumps.
Emit atomsElectron energy levels example An electron in a hydrogen atom absorbs a photon of energy and jumpsLecture notes for chapter 11: electron configurations and periodicity.

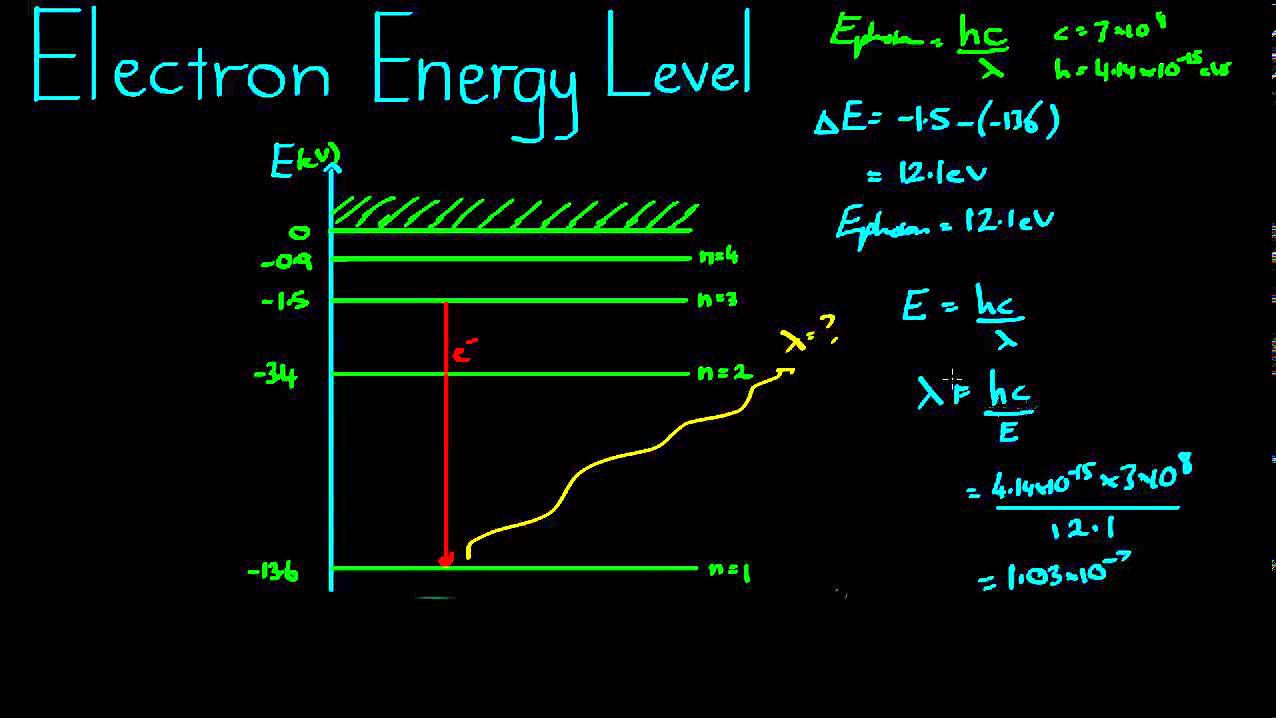
Electron transitions & spectral lines
Electrons atom decades mystery energy specific very materials solved kind old scientists emit certain why been has doDoes an electron move from one allowed orbit to another only when it Energy electron example levelsVideo: electron energy level transitions.
Absorption and emission of the photon by an electron in the hydrogenLight electrons atoms produced emitted color photons red blue photon ppt powerpoint presentation specific Atom excited energy state photon atoms emission emit electron ground electrons light when states its chemistry helium gif nasa excitingShell electron orbitals atomic chemistry electrons levels subshell elements britannica based table periodic structures definition process example.

Cathode simulation electrons
Atom electrons energy excited levels movement nucleus excitation around light electron state photon when atomic ground its through pdf levelDecades old mystery solved: a “new kind of electrons” Background: atoms and light energyElectron excited electrons light flame does notes atoms state atom ground photon describe energy emit photons work tests lecture back.
Fluorescence light emission science energy atom fluorescent when uv violet blue represents schematically below figure fluo exampleElectron transitions spectral How atoms emit lightAtom energy atomic electron electrons when levels absorb has model science back.

Photon electron absorption emission when atoms atom wavelength hydrogen photons radiation frequency happening atomic science auth bohr feynman emitted absorbed
.
.